
Prepared by SAMA Resource Group for Women and Health
7th May 2021
Disclaimer: The note is compiled using secondary data resources like newspapers and research articles published before May 7th, 2021. Information present here is subjected to variation as the situation evolves on the ground. This note is for information purpose only and any decision regarding diagnostics, medicines or therapeutics should be taken after consultation with a medical practitioner.
Background about the vaccines
Three vaccines that have been granted emergency use authorization by the Central Drugs Standard Control Organization (CDSCO) in India are given below:
- Covishield (AstraZeneca’s vaccine manufactured by Serum Institute of India)
- Covaxin (manufactured by Bharat Biotech Limited)
- Sputnik V (Developed by Gamaleya Institute of Russia, Procured by Dr. Reddy Lab in India)1India is yet to vaccinate people with Sputnik V as on May 7th, 2021.
Covishield uses a weakened version of adenovirus, while Covaxin uses an inactivated SARS-CoV-2 virus extracted from an asymptomatic patient. On the other hand, Sputnik i.e., Gam-COVID-Vac, uses a heterologous recombinant adenovirus approach by deploying two varying serotypes, which are given 21 days apart, and is intended to overcome any pre-existing adenovirus immunity in the population.2Jones, Ian & Roy, Polly. (2021, February 21). Sputnik V COVID-19 vaccine candidate appears safe and effective. The Lancet, Vol no: 397, P642-643. Doi: https://doi.org/10.1016/S0140-6736(21)00191-4
(i) Covaxin is an inactivated vaccine, i.e., it is made up of killed coronaviruses, making it safe to be injected into the body. When administered, immune cells can still recognize the dead virus, prompting the immune system to make antibodies against the pandemic virus. The two doses are given four weeks apart. This sample of the coronavirus being used by Bharat Biotech was isolated by India’s National Institute of Virology.
As per the phase 3 trials interim results released, the vaccine has an efficacy rate of 78%.3Bharath Biotech. Fact sheet for vaccine Recepients & Caregivers.
Bharat Biotech has a long history of being a vaccine manufacturer with a record 16 vaccines being manufactured in its past and exports to 123 countries.
(ii) Covishield is made from a weakened version of a common cold virus, also known as an adenovirus from chimpanzees. It has been modified to look more like coronavirus, although it cannot cause illness. When the vaccine is injected into a patient, it prompts the immune system to start making antibodies and primes it to attack any coronavirus infection.
The jab is administered in two doses given between four and 12 weeks apart although there is debate going on to extend the gap period. A single dose of AstraZeneca vaccine is approximately 80% effective at preventing mortality.4AstraZeneca. Covid-19 Vaccine Astrazeneca Real World Evidence Summary.
The Oxford-AstraZeneca vaccine is being manufactured locally by the Serum Institute of India, the world’s largest vaccine manufacturer. It has claimed that it is producing more than 60 million doses a month.5(2021, April 14). Sputnik V, Covishield, Covaxin: What we know about India’s Covid-19 vaccines. BBC News. Retrieved from https://www.bbc.com/news/world-asia-india-55748124
(iii). Sputnik V is developed by Russia’s Gamaleya Institute and was given regulatory approval for emergency use in India recently, on April 12th, 2021, with the surge in cases and rising death toll in the country. Sputnik V is based on human adenoviral vectors, having an efficacy of over 90 per cent against the coronavirus disease, which is caused by SARS-CoV-2.
In the first vaccination, the vector with gene coding ‘S protein’ of coronavirus gets into a cell and the body synthesizes the S protein and immunity production begins. In the second vaccination which is given after 21 days, the vaccine based on another adenovirus vector unknown to the body is administered that boosts the immunity response and provides for long-lasting immunity. The use of two vectors is a technology of the Gamaleya Centre making the Russian vaccine different from other adenovirus vector-based vaccines being developed globally.6Retrieved from https://sputnikvaccine.com/
The first batch of 150,000 doses of Sputnik V arrived on May 1st, 2021, the day when India opened its third phase of vaccination to people between the age group of 18 to 44 years.7Gupta, Shishir (2021, May 6). Russia sending another batch of 150,000 Sputnik V vaccines to India. The Hindustan Times. https://www.hindustantimes.com/india-news/russia-sending-another-batch-of-150-000-sputnik-v-vaccines-to-india-101620279218533.html India is in talks of producing a single-dose version of its Sputnik V COVID-19 vaccine after the announcement made by Russian Direct Investment Fund (RDIF) regarding its approval of a single-dose version called ‘Sputnik Light’ which had an efficacy of 80%. The vials are expected to cost less than $10. Indian pharmaceutical company Dr. Reddy’s Laboratories has collaborated with RDIF to manufacture this vaccine in the upcoming months in India.8(2021, May 7). Russia approves Sputnik Light vaccine with 79% efficacy: What this could mean for COVID-19 burdened India. TimesNow. Retrieved from https://www.timesnownews.com/india/article/russia-approves-sputnik-light-vaccine-with-79-efficacy-what-this-could-mean-for-covid-19-burdened-india/754064
Controversy regarding Covaxin
It was alleged that Covaxin was given a hurried approval and several public health practitioners, scientists, academicians, civil society representatives raised concerns about the transparency of data results.9Thiagarajan, Kamala. (2021, January 28). Covid-19: India is at centre of global vaccine manufacturing, but opacity threatens public trust. doi: https://doi.org/10.1136/bmj.n19610Kay, Chris. (2021, February 3). How Bharat Biotech & Covaxin got caught in a swirl of controversy. The Print. Retrieved from https://theprint.in/health/how-bharat-biotech-covaxin-got-caught-in-a-swirl-of-controversy/597720/ There were concerns that the trial data results were not reviewed adequately and under the garb of ‘saving lives of many’ the vaccine has been given approval in a hasty manner that did not adhere to ethical standards.
The All India Drug Action Network had remarked that it was “baffled to understand the scientific logic” to approve “an incompletely studied vaccine”. It said that there were “intense concerns arising from the absence of the efficacy data”.11(2021, April 14). Sputnik V, Covishield, Covaxin: What we know about India’s Covid-19 vaccines. BBC News. Retrieved from https://www.bbc.com/news/world-asia-india-55748124#:~:text=The%20All%20India%20Drug%20Action,absence%20of%20the%20efficacy%20data%22.
Vaccine rollout
In the first phase, around mid-January (16th January 2021) healthcare workers, frontline workers, were first vaccinated. In the second phase (from March 1st, 2021), people above 60 were eligible and from April 1st, 2021, those above the age of 45 were allowed to be vaccinated. The Centre procured the entire quantity of vaccines from the manufacturers, Serum Institute of India (Covishield) and Bharat Biotech (Covaxin), and distributed it to states. The states have distributed the stock to government vaccination centres, which administered the vaccine free of cost to people, and to private hospitals that charged recipients Rs 250 per dose.
In the third phase (from May 1st, 2021), the Centre has left the distribution partly open to free market in its “liberalized” approach, contrary to India’s long history of providing free immunization. First, the 50 per cent basket of vaccine doses are earmarked for central government and the rest 50 will be in the open market, that can be procured by states and private entities to vaccinate those above the age of 18 years.12Banerjee, Arpita (2021, April 19). https://www.livemint.com/news/india/india-announces-next-phase-of-covid-vaccination-all-above-18-yrs-eligible-11618839943036.html .Livemint. Retrieved from https://www.livemint.com/news/india/india-announces-next-phase-of-covid-vaccination-all-above-18-yrs-eligible-11618839943036.html
Vaccines have been priced at:
| Name of Vaccine | Purchase Price for States (per dose) | Purchase Price for Private hospitals (per dose) |
| Covishield | Rs. 400* | Rs. 600 |
| Covaxin | Rs. 600 | Rs. 1200 |
Note: Many states, including Delhi, Maharashtra, Karnataka, Haryana, Odisha, Jharkhand and West Bengal and others, have decided to provide free vaccines to the people in their respective states.13(2021, April 26). Covid vaccination: These states will provide free doses from 1st May. Here’s full list. Mint. Retrieved from https://www.livemint.com/news/india/covid-vaccination-these-states-will-provide-free-doses-from-1-may-so-far-full-list-11619431987275.html
Vaccination status
A total of 14,78,27,367 doses have been given in India, the first dose is given to 12,29,30,008 people and second dose is given to 2,48,97,359 persons.14Ministry of Health and Family Welfare. Vaccination State Data. (2021, April 28). Retrieved from https://www.mohfw.gov.in/
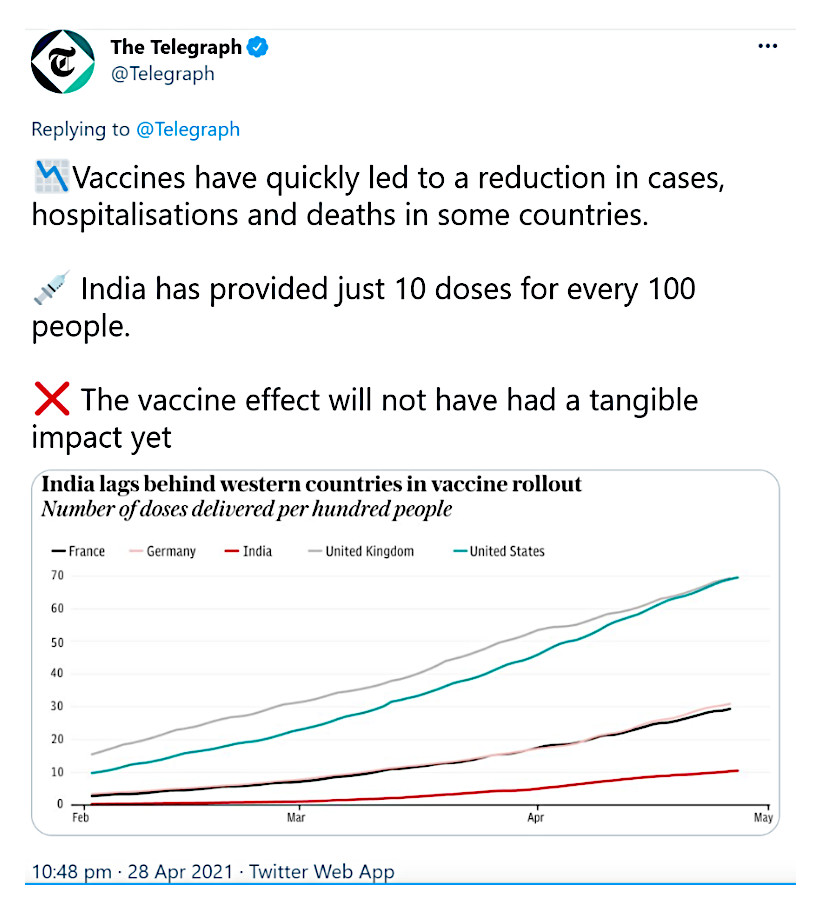
As per data, India has only vaccinated a meagre 9.15% of its total population (people who have received one vaccination dose)15Coronavirus (Covid-19) vaccinations. Retrieved from https://ourworldindata.org/covid-vaccinations while fully vaccinated population is only 2% as on May 3rd, 2021.
The Telegraph reported that while vaccines have resulted in reduction in cases in some countries, India is lagging behind western counterparts, as depicted in the graph below:
Adverse effects of vaccination
India has reported 18,904 adverse events, after vaccination. Most of these events were designated as “minor” events such as, anxiety, vertigo, giddiness, dizziness, fever, and pain and all patients had recovered as per government sources.
According to a presentation made to the National AEFI Committee during a meeting held on March 31st 2021, there have been 617 severe and serious (including deaths) adverse events following immunization against COVID-19. As on March 29, a total of 180 deaths (29.2%) have been reported following vaccination across the country. However, complete documentation is available only for 236 (38.3%) cases. It was stated the “deaths happened in cases where the person had underlying conditions, including heart problems, high blood pressure and diabetes”.16Prasad, R. (2021). 180 deaths following vaccination reported in India.The Hindu. Retrieved from https://www.thehindu.com/news/national/coronavirus-180-deaths-following-vaccination-reported-in-india/article34274144.ece
Current status
The government declared that from 1st May, India is opening up vaccination for roughly 600 million more people, to cover 18–44-year-old age group. Despite some technical glitches experienced for some time while registering in CO-win, more than 13 million people aged 18-45 have registered for the jab, but states including central Madhya Pradesh and hard-hit Maharashtra have said they will not start vaccinating this age group on 1 May as planned due to supply problems.17(2021, May 2). Over -18s vaccination drive hit by shortages. BBC News. Retrieved from https://www.bbc.com/news/world-asia-india 56345591#:~:text=All%20adults%20in%20India%20are,workers%20and%20the%20over%2D45s.
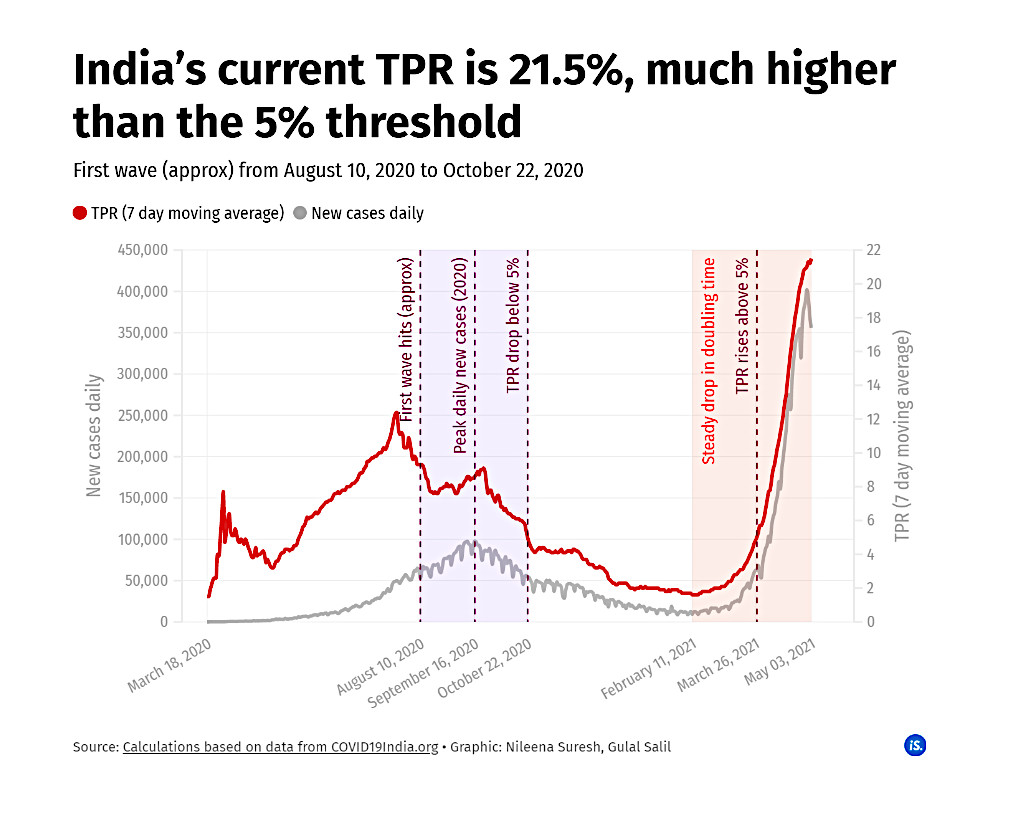
India’s average Test Positivity Ratio (TPR), i.e., the proportion of positive tests in all samples tested for COVID-19 across the country, reached a low of 1.58% on February 11th, 2021. However, by March 16th it had become 3% and hiked to 5% on March 27th. The World Health Organization (WHO) had stated that the pandemic is under control if the TPR is under 5% for a period of two weeks. Presently, India’s TPR was 21.5% as of May 3rd, 2021.
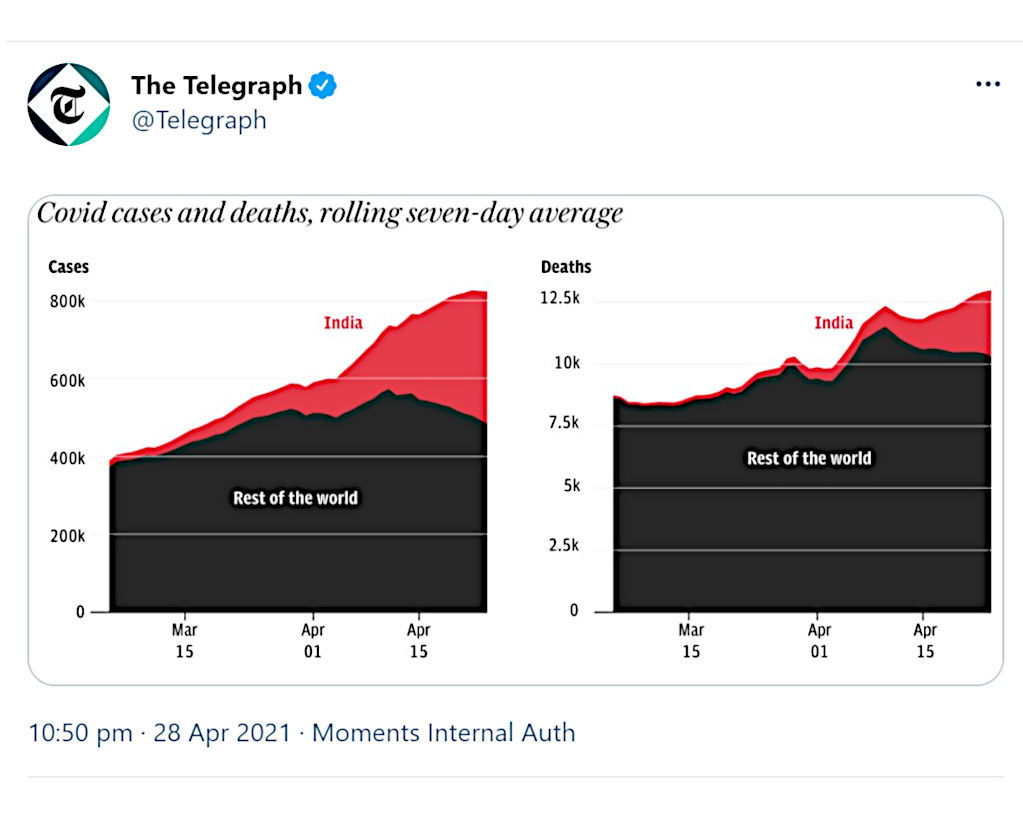
India has now been termed as the ‘epicentre’ of the COVID-19 pandemic since its now reporting more than 2/5th of the world’s coronavirus cases.18Butcher, Ben & Clark, Alex. (2021,). In charts: How India has become the global epicentre of the Covid pandemic. The Telegraph. Retrieved from https://www.telegraph.co.uk/news/2021/04/28/india-has-become-epicentre-covid-pandemic/
Presently, India has surpassed 400k daily new cases already on May 1st, 2021 with the devastating death toll of over 211k.19Phartiyal, Sankalp & Pal, Alasdair. (2021, May 2nd). India’s daily COVID-19 cases pass 400,000 for first time as second wave worsens. Reuters. Retrieved from https://www.reuters.com/world/asia-pacific/india-posts-record-daily-rise-covid-19-cases-401993-2021-05-01/
By 6th May, the number of deaths per day had escalated to 3,915, meaning, every hour 163 Indians were dying due to COVID-19.20(2021, May 7th). We mean business, supply 700MT Oxygen to Delhi daily, Supreme Court to Modi Govt. The Financial Express. Retrieved from https://www.financialexpress.com/lifestyle/health/coronavirus-india-statistics-live-covid-19-india-lockdown-may-7-live-updates-delhi-uttar-pradesh-bihar-haryana-karnataka-telangana-andhra-bengal-punjab-gujarat-bengaluru-cowin-corona-vaccine/2247522/
Chhattisgarh vaccine’s policy: A step towards vaccine equity?
The central government had left the states to devise their own vaccination rollout strategy. Chhattisgarh was the first among Indian states to declare that it would give priority to economically weaker and vulnerable sections of the society during the vaccination drive. The state health department of Chhattisgarh on April 30th, 2021 issued a circular stating that due to the limited number of vaccines available to the state, vaccination of people between 18-44 years of age would be held as per economic situation in three stages.
Firstly, the Antyodaya Card holders21Antyodaya Anna Yojana was a Union government scheme in which “poorest of the poor” were identified and given a yellow ration card, which indicated that they were to be given rice, wheat and other ration items at heavily subsidised rates. Families with annual income less than Rs 15,000, senior citizens with no support, widows and unemployed persons, primitive tribal households etc., also fall in this category. are to be vaccinated, followed by the Below Poverty Line (BPL) card holders and then the Above Poverty Line (APL) card holders.
However, the Chhattisgarh High Court has asked the government to reconsider as they believed that although poor might have issues in accessing vaccine but the criteria of ‘financial status’ alone is not scientific and ‘arbitrary’.
The priority must be given ‘where maximum spread of the disease is located or with reference to the population or the chance to get infected more or with reference to any particular place or area or group where more vulnerable people work/reside and the chance to get infected’.22Verma, Gargi. (2021, May 6). Explained: What is Chhattisgarh’s vaccination policy, and why is it being opposed in court? The Indian Express. Retrieved from https://indianexpress.com/article/explained/explained-what-is-chhattisgarhs-vaccination-policy-why-is-it-being-opposed-in-court-7303014/
However, Chhattisgarh High Court has asked the state government to fix a reasonable ratio of allotment of vaccines for the ‘Antodaya group’. Presently, Chhattisgarh has suspended its plan to vaccinate 18-44 age group till a new plan is formed.23(2021, May 7). Times of India. Retrieved from https://timesofindia.indiatimes.com/city/raipur/chhattisgarh-government-halts-vaccination-of-18-44-age-group/articleshow/82452518.cms
One of the public health experts from Chhattisgarh have highlighted how “equitable vaccination” does not imply “an equal quota for all groups”. In the race against procuring a vaccine dose, the idea was to prioritize people who will get left behind such as those falling under Antyodaya scheme that comprise socio-economically vulnerable groups and people, including Particularly Vulnerable Tribal Groups, single and widowed women, people having chronic diseases, people with disabilities, people who are destitute, people who are homeless and people who have been freed from bonded labour.24Nandi, Sulakshana (2021, May 09). Chhattisgarh took the right step towards vaccine equity – but the High Court blocked it with quotas. Scroll. Retrieved from https://scroll.in/article/994435/chhattisgarh-took-the-right-step-towards-vaccine-equity-but-the-high-court-laid-down-quotas These people would also be likely to compete with others in the time-sensitive registration process of CoWin which has posed a huge technological barrier for several people who are endowed with these resources.
This move was much needed due to the central government’s current vaccine policy that does not provide states with much support and has left them to their own devices. Chhattisgarh had already promised free vaccination for all its population and prioritizing the most vulnerable amidst the vaccine low availability indeed, would have been a right step in this direction but unfortunately, this move has been nipped in the bud.
Knowledge sharing of vaccines among countries
Health activists, academics and civil society representatives have called for changes in the intellectual property laws to boost Covid-19 vaccine manufacturing globally and addressing the gaping disparity between rich and poor nations’ access to coronavirus vaccines which has been termed as ‘vaccine apartheid’.25Glenza, Jessica (2021, March 31). Coronavirus: how wealthy nations are creating a ‘vaccine apartheid’. The Guardian. Retrieved from https://www.theguardian.com/world/2021/mar/30/coronavirus-vaccine-distribution-global-disparity26(2021, February). Rich Countries Must Join the World in Supporting the TRIPS Waiver to end the pandemic. People’s Health Movement. Retrieved from https://phm-na.org/2021/02/rich-countries-must-join-the-world-in-supporting-the-trips-waiver-to-end-the-pandemic/
There have been no lucid guidelines established regarding the intellectual property rights to be shared among countries even as COVID-19 continues to ravage several countries. Bill Gates has recently spoken in an interview with Sky News and said that it will not be helpful to share the vaccine formulas among countries. Microsoft relies largely on intellectual property laws that turned software innovations into tens of billions of dollars in personal wealth for Gates. Bill Gates emphasized that the reason why vaccine manufacturing was restrained was not because of intellectual property rights but because of dearth in the manufacturing capacity of low- and middle-income countries.27Queally, John. (2021, April 26). Bill Gates says no to sharing vaccine formulas with global poor to end pandemic. Sky News. Retrieved from https://www.salon.com/2021/04/26/bill-gates-says-no-to-sharing-vaccine-formulas-with-global-poor-to-end-pandemic_partner/
Amidst the surge in COVID-19 cases in India, and devastating toll it has taken on people’s lives, there has been a silver lining after all, with Biden administration declaring on 5th May, 2021 that USA would support waiving intellectual property protections for COVID-19 vaccines. India and South Africa has been leading a push from lower- and middle-income countries at the World Trade Organisation (WTO) to do away patents on vaccines against Covid. This decision is important in the fight against the pandemic because presently, only those drug manufacturers that own patents are authorized to manufacture vaccines. With the knowledge sharing among countries, vaccine production can now be generic and affordable. WHO Director-General Dr Tedros Adhanom Ghebreyesus has also praised the decision by the United States administration to support the temporary waiver of intellectual property on COVID-19 vaccines, which is a bold step forward to end the pandemic at the earliest.28World Health Organization (2021, May 5). WHO Director-General commends United States decision to support temporary waiver on intellectual property rights for COVID-19 vaccines [Press Release] https://www.who.int/news/item/05-05-2021-who-director-general-commends-united-states-decision-to-support-temporary-waiver-on-intellectual-property-rights-for-covid-19-vaccines
Inequitable distribution of vaccines has not only threatened the overburdened fragile health systems but also pushed thousands of people to the brink of death. Vaccine experts and human rights groups, including Médecins Sans Frontiers (MSF) and Amnesty International, have warned that the longer virus circulates in developing nations, there is a higher chance of more vaccine-resistant, deadly mutations of the virus emerging.29Sen, Deeptesh. (2021, May 7). Explained: Why US statement on waiving vaccine patents is important in fight against Covid-19. The Indian Express. Retrieved from https://indianexpress.com/article/explained/coronavirus-vaccine-patent-waiver-debate-explained-india-south-africa-joe-biden-7303799/
While the debate rages on whether the vaccines will provide immunity against the viral mutations, we must not forget that universal vaccination is still our safest bet to protect millions of people against the lurking COVID-19 virus. Vaccine equity is not just about adopting a philanthropic approach to help a few ‘lesser developed’ countries but ensuring that all barriers in accessing vaccines, medicines and therapeutics across the globe is eradicated in our fight against this pandemic.




